
#LINE EMISSION SPECTRUM OF HYDROGEN SERIES#
Its spectrum consists of a series of spectral lines. Give an account of the spectral series of hydrogen atoms. Hence, from the above hydrogen spectrum, the series which lies in the ultraviolet region is the Lyman series. the electronic transitions corresponding to these series shown in the below diagram, Such series were identified in the course of spectroscopic investigations and are known as the Lyman, Balmer, Paschen, Brackett, and Pfund series. For the hydrogen spectrum, the results of the Bohr’s model suggested the presence of other series spectra for hydrogen atoms corresponding to transitions resulting from nf=1 and ni=2,3,etc.

Since both nf and ni are integers, this immediately shows that in transitions between different atomic levels, light is radiated in various discrete frequencies. The Lyman series is in the ultraviolet region.Īccording to third postulate of Bohr’s model, when an atom makes a transition from the higher energy state with quantum number ‘ni’ to the lower energy state with quantum number nf(nf
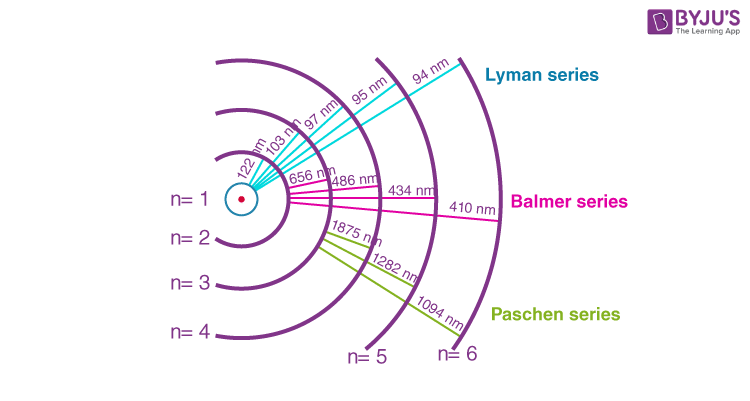
For the Brackett series, n 1=4, n 2=5,6,7.Also, n 1 and n 2 are whole numbers and for a particular series, n 1 is constant and n 2 varies. Here, R is a constant called the Rydberg constant, and its value is equivalent to 109677 cm−1. The equation provides a calculation of the wavenumber v of the lines by the formula: However, in 1890, Rydberg gave a very simplified theoretical equation for the wavelength of these lines. Transition from the fifth shell to any other shellĪ large number of spectral lines are present in the hydrogen spectrum. Transition from the fourth shell to any other shell Transition from the third shell to any other shell Transition from the second shell to any other shell Transition from the first shell to any other shell


 0 kommentar(er)
0 kommentar(er)
